
|
|
Descent Into the Ice
|

|
|
Classroom Activity
|
Objective
To investigate the phase change of water turning into ice.

-
copy of the "Is It Icy Water or Watery Ice?" student handout (PDF
or
HTML)
-
copy of the "Experiment Data Sheet" student handout (PDF
or
HTML)
-
alcohol thermometer with a low point of -10º C (14º F) or less
- 2 150-mL beakers
- 20-mL test tube
- 8-oz foam coffee cup
- 16-oz foam coffee cup
- crushed ice
- ice water
- room-temperature water
- salt
- spoons: teaspoon, tablespoon, plastic spoon
- stopwatch
- paper towels
- graph paper

-
Understanding water and ice helps scientists study glaciers.
Some glaciers contain water wells. Tell students that in this
activity, they are going to investigate how ice and water can
coexist.
-
Organize students into teams and provide each team with a copy
of the student handouts and other materials. Have teams appoint
one person to run the experiment, one to be the timekeeper, and
one to be the recorder.
-
Have students prepare ice/salt baths and run the experiment
according to directions on their handouts. The student
performing the test will pull out the test tube once every
minute to check for ice formation. Make sure students do this as
quickly as possible so the air does not warm the water in the
test tube. Students should run the experiment for 12 minutes.
-
When students are ready to clean up, have them put the test tube
in warm water to melt the ice and free the thermometer.
-
Ask students to graph their results with time on the
x-axis and temperature on the y-axis. What did
they find? What do the results show about how water turns into
ice and why the two may be able to coexist simultaneously?
-
As an extension, repeat the experiment but reverse the
procedure. Have students place a thermometer that has been
frozen in a test tube in a cool water bath and plot the
temperature change as the ice in the test tube melts into water.
How do student graphs compare to those in the initial
experiment?

In this activity, students see ice and water coexist. It helps them
understand how glaciers can have interior lakes.
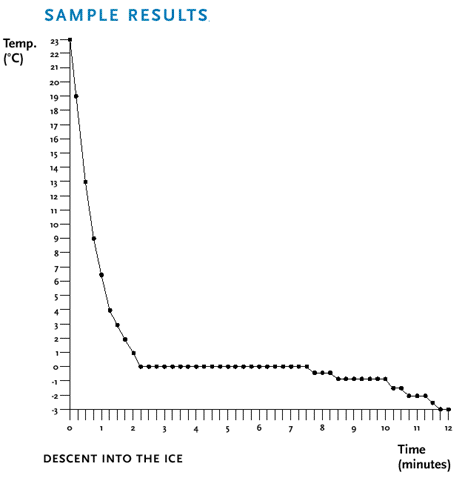
Students will observe that the temperature of the water drops, then
remains constant as the water and ice coexist, and then drops again
once the water has turned into ice.
The following process occurs: When energy (heat) is transferred from
the water in the test tube to the ice/salt bath mixture, the
temperature in the test tube starts to fall. At 0º C, ice
crystals begin to form. At this time, the water must lose an
additional amount of energy to form ice crystals. The time required
to lose this added energy is indicated by the flat section of the
graph. During this period, all of the energy is being spent on
changing the state of matter (water into ice) and not on changing
the temperature. Any decrease in temperature initiates more ice
crystal formation, which in turn releases energy that prohibits the
temperature from falling below 0º C. Only after all of the
water has turned to ice will the temperature again begin to drop.
Students may find that their water freezes at a temperature other
than 0º C. This is likey due to thermometer
inaccuracy—many thermometers have an uncertainty of plus or
minus one or two degrees.
Students may think that their water is frozen, when in fact only the
outside edge is frozen. Remind them that as in a natural body such
as a lake, the surface freezes first. The sides and bottom also
freeze first because in this experiment they are in closest contact
with the ice.
If students started out with more water in their test tubes, the
flat section on the graph would extend, as there would need to be
more heat lost before all of the water could freeze.

Web Sites
NOVA Web Site—Descent into the Ice
www.pbs.org/nova/mtblanc/
In this companion Web site for the NOVA program, find out how
Earth's glaciers are holding up, learn about glacial hazards
worldwide, explore an ice climber's gear, and follow the life cycle
of a glacier.
Glacier
www.glacier.rice.edu/
Provides background information on glaciers and shows what it is
like to work at a scientific research center in Antarctica.
The Glacier Story
nsidc.org/glaciers/story/page1.html
Tells how a glacier forms, moves, and retreats.
Book
Hambrey, Michael and Jürg Alean. Glaciers. Cambridge,
England: Cambridge University Press, 1992.
Discusses how glaciers form and move, outlines the different types
of glaciers, and provides photographs of glaciers around the world.

The "Is It Icy Water or Watery Ice?" activity aligns with the
following National Science Education Standards.
Grades 5-8

|
Science Standard B:
Physical Science
|

|
Properties and changes of properties in matter:
-
A substance has characteristic properties, such as density, a
boiling point, and solubility, all of which are independent of
the amount of the sample.
Grades 9-12

|
Science Standard B:
Physical Science
|

|
Structure and properties of matter:
-
Solids, liquids, and gases differ in their distances and angles
between molecules or atoms and therefore the energy that binds
them together. In solids the structure is nearly rigid; in
liquids molecules or atoms move around each other but do not
move apart; and in gases molecules or atoms move almost
independently of each other and are mostly far apart.
Classroom Activity Author
James Sammons has taught middle and high school science for 30
years. His teaching practices have been recognized by the National
Science Teachers Association, the Soil Conservation Service, and the
National Association of Geoscience Teachers.
|

|






