
|

|

|
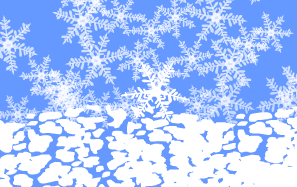
High in the atmosphere, molecules of water vapor cling to a
tiny dust particle, arranging themselves in a crystalline
structure. Other molecules of water vapor bind to the crystal,
forming the familiar six-sided snowflake.
|

|

|
|

|
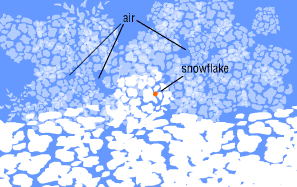
On the slopes of a mountain, the snowflake lands on a glacier.
As countless other flakes begin to weigh down on it, the
snowflake's delicate points start to break off.
Here on top of the glacier the snow is about 90 percent air.
|

|

|
|

|
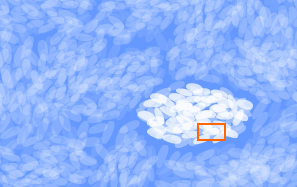
As time passes, subsequent snowfalls cover the snowflake. The
weight of the overlying layers compacts the snow surrounding
the flake, causing these flakes to nestle closer to one
another.
The flake's points slowly evaporate as individual water
molecules leave the more pointed areas and redeposit
themselves in the air spaces. The same happens to the
surrounding flakes. Gradually, all these flakes become small
grains of ice.
By now the snow is about 50 percent air.
|

|

|
|

|
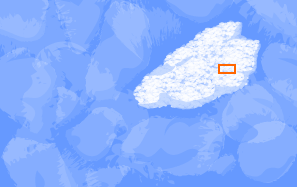
Year after year, more snow falls on the glacier. As the snow
accumulates, the ice crystals surrounding the flake become
more compressed and the air spaces between the crystals
shrink.
The crystals eventually merge into larger crystals, trapping
air spaces that were between them inside bubbles. When
temperatures are warm and some melting occurs, the change from
snow to glacial ice can be very rapid, as short as a single
year.
The ice, now about 10 percent air, is as dense as it will get.
|

|

|
|

|
The snowflake merges with a larger ice crystal deep under the
glacier's surface. Succumbing to the tremendous pressure
caused by the weight of the ice above, the crystal is making
its long journey down what is essentially a river of ice.
|

|

|
|

|
In extremely cold regions, a glacier's ice is often frozen to
bedrock, which hampers its flow. This type of glacier is known
as a "cold glacier."
With "warm glaciers," the weight of the ice creates a thin
film of liquid water between the ice and the land, in the same
way that the weight of an ice skater causes the blade of an
ice skate to melt the ice, forming a thin layer of water. As
with the skate, the water acts as a lubricant, allowing the
glacier to slide over the land more easily.
|

|

|
|

|
As it meanders through a valley, the moving glacier carries
the crystal along with it. Small glaciers move slowly—10
feet or less in a year. Large glaciers, powered by the weight
of a lot of ice, can travel half a mile or more in a year.
How fast the ice flows also depends on whether it's part of a
warm glacier or a cold glacier—warm glaciers typically
flow faster than cold glaciers. Other factors include the
temperature of the ice and the pitch of the slope it's sliding
down.
|

|

|
|

|
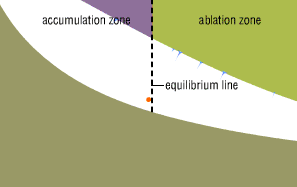
The crystal is now at the glacier's so-called equilibrium
line—the line that divides the area where the glacier
gains more mass than it loses from the area where it loses
more mass than it gains.
|

|

|
|

|
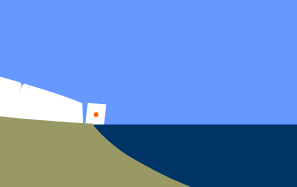
The crystal has reached the end of its journey. The ice
surrounding it has calved from the glacier and is now floating
inside an iceberg. The iceberg will eventually melt, releasing
the water molecules that entered the glacier as a snowflake
into the ocean. There, through evaporation, they will
ultimately return to the atmosphere, thus closing the cycle.
Glaciers that don't meet a lake or the sea lose mass by
melting or by sublimation, a process in which water changes
directly from solid to gas, skipping the liquid stage.

|

|

|

|

