Einstein's Big Idea
|  |
Student Handout
|
Planning Your Trip
Procedure
Read your "Reaction Worksheet" handout carefully. For your trip to Pluto, you will be riding in a spacecraft that has a
mass of 135,000 kilograms. Most of the mass is in the rocket boosters, which
are needed for taking off and escaping Earth's gravitational field. Your task
is to travel to Pluto, land on its surface, take samples of the surface ice and
rocks, and then return to Earth. To accomplish this trip, you will require a
lot of energy-a total of 8 x 1032 electron Volts (eV)! Your cruising
velocity will be 12.0 kilometers per second (a speedy 27,000 miles per
hour!). Refer to the equations on your "Reaction Worksheet" handout to obtain the
amount of energy released per molecule burned (or reaction occurred) for each
of your fuels. Write these in the tables below. To find the number of reactants
(or reactions) you need for your round trip, divide your total round trip
energy by the energy released per molecule (or reaction) for each fuel
type/process listed. Record your results.
Sample calculation for wood

To determine the total mass (g) of fuel required to make the trip, multiply
the number of reactants (or reactions) needed for the round trip by the mass in
grams per molecule (or reaction) for each fuel type/process listed. Record your
results.
Sample calculation for wood
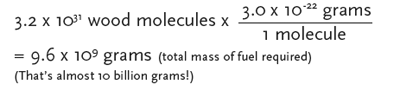
|
Fuel Type
|
Mass (g) per Molecule
|
Energy Released per Molecule (eV)
|
# Reactants Need for Round Trip
|
Total Mass (g) of Fuel Required
|
|
Wood
|
3.0 X 10-22
|
25 eV
|
3.2 X 1031
|
9.6 X 109
|
|
Coal
|
2.0 X 10-23
|
|
|
|
|
Natural Gas
|
2.7 X 10-23
|
|
|
|
|
Gasoline
|
1.9 X 10-22
|
|
|
|
|
Fuel Process
|
Mass (g) per Reaction
|
Energy Released per Reaction (eV)
|
# Reactions Need for Round Trip
|
Total Mass (g) of Fuel Required
|
|
Fission
|
4.0 X 10-22
|
|
|
|
|
Fusion
|
1.7 X 10-23
|
|
|
|
|
Photon drive
|
3.4 X 10-24
|
|
|
|
Questions
Write
your answers on a separate sheet of paper.
What do all the reactants of wood and fossil fuels have in common? Compare the products of wood and fossil fuel reactions with the products of
nuclear reactions. How are they the same? How are they different? Compared to pure uranium fission, how many times more wood would you have to
burn to make the trip to Pluto? How many times more wood compared to a photon
drive engine? If
Pluto is 5.9 x 109 kilometers from Earth, how long will it take you,
in years, to make the trip to Pluto and return home? (Assume a straight line, a
constant velocity with no deceleration or acceleration, and a speed of 12.0
kilometers per second.)
|