
Build a Steroid
As Percy Julian knew only too well, the synthesis of a
chemical compound found in nature is a highly complicated
process that can take years of research and experimentation.
Beginning with a readily available "starter" chemical like
diosgenin, which can be extracted from the cheap and plentiful
Mexican yam, makes a chemist's job easier. This compound
already has the basic chemical structure that all major
steroids share. Though it may take numerous chemical reactions
to get there, chemists can fashion diosgenin into any steroid,
from estrogen for menopausal women to anabolic steroids for
athletes. In this feature, watch as diosgenin transforms into
adrenal hormone cortisone.—Claudine Ko

|
|
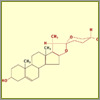
Diosgenin
The basic molecular structure of a steroid includes four
rings—three six-membered and one
five-membered—which are built entirely of carbon
atoms. The biological function of a steroid depends on
the groups attached to these rings. Diosgenin, for
instance, has two additional rings (highlighted here in
red). To modify diosgenin's molecular structure to the
desired cortisone, our virtual chemist needs to perform
a series of chemical reactions.
|

|
|
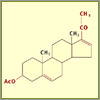
Intermediate Compound #1
Multiple steps have been combined to give us our first
intermediate compound (16-Dehydropregnenolone acetate,
shown here). Note how the red groups have changed. The
next steps include oxidation reactions, which add oxygen
to the compound. In this case, the chemist uses chromium
trioxide (CrO3) and then heats the mixture,
which breaks bonds and allows the oxygen to recombine
with the steroid.
|

|
|
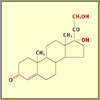
Intermediate Compound #2
After several more steps, the chemist reaches another
intermediate compound (11-Dehydrocorticosterone, shown
here). The chemist must be precise to prevent any side
reactions, which could result in unwanted compounds. To
verify that the correct intermediate compound was
produced, Percy Julian might have checked its melting
point, which is unique to each compound; today chemists
use various forms of spectroscopy.
|

|
|
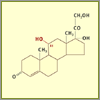
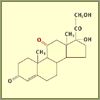
Hydrocortisone
Julian made a major chemical breakthrough when he
figured out how to add a hydroxyl group at the difficult
carbon-11 position, turning the previous intermediate
compound into hydrocortisone. But the process took 27
steps. Later, biochemists discovered a fungus that could
add the hydroxyl group in a single step.
|

|
|
Cortisone
To produce cortisone, the chemist needs only modify the
hydroxyl group in hydrocortisone using another oxidation
reaction. When it was first isolated from animals,
cortisone—which is used to treat ailments such as
rheumatoid arthritis, asthma, and hepatitis—cost
about $100 a gram. But after chemists determined how to
make it from the Mexican yam, production increased,
prices dropped, and the drug became widely available.
|
|


We recommend you visit the
interactive version. The text to the left is provided for printing purposes.
|


|
|
Forgotten Genius Home |
Send Feedback |
Image Credits
|
Support NOVA
|
© | Created
January 2007
|






