

|
|
|

|
The Structure of Metal Heat 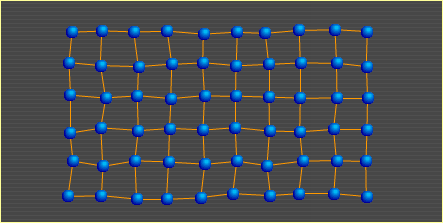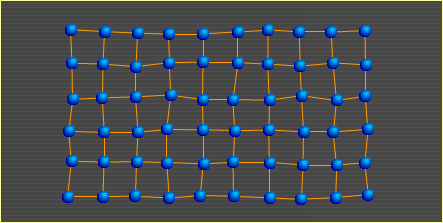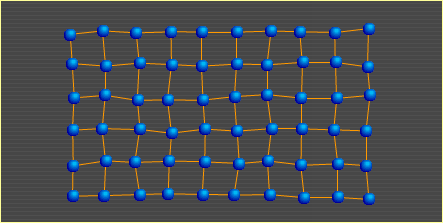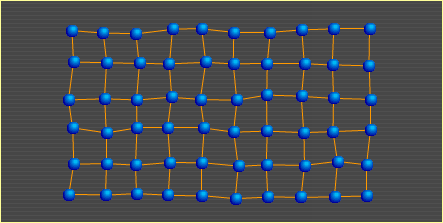
Heat is nothing more than atoms (and molecules) in motion. The faster an atom moves around, the more heat it carries. In the diagrams presented in the other three sections, the points show where atoms would lie if there were no motion due to heat; that is, if the metal's temperature were at absolute zero (-273°C). The atoms in this diagram do show temperature-related motion. As heat in a metal increases, its atoms move faster. At some point, they move so fast that the bonds holding them to surrounding atoms begin to break. The breaking of bonds causes the metal to soften. When 10% of the bonds are broken, the metal is in the liquid state. Metal Basics | Move | Slip | Bend | Heat
Towers of Innovation | The Collapse | Above the Impact Outfitting Firefighters | The Structure of Metal | Letters | Resources Transcript | Site Map | Why the Towers Fell Home Search | Site Map | Previously Featured | Schedule | Feedback | Teachers | Shop Join Us/E-Mail | About NOVA | Editor's Picks | Watch NOVAs Online | To Print PBS Online | NOVA Online | WGBH © | Updated May 2002 |