|
Color photos, old newspapers, the Declaration—we've all seen
materials damaged by long-term exposure to light. But what's
actually happening?
The Declaration of Independence took a lot of abuse in the first
decades of its life. It was rolled and unrolled countless times. It
was tossed in a trunk or stuffed in a linen bag and whisked away
every time the British Army got too near. And for 35 years,
beginning in 1841, it was hung on a wall in the Patent Office in
Washington, D.C., across from a sunny window.
The harm this last insult caused is apparent in a quote from the
Public Ledger in 1876, when the Declaration was taken off
that wall and transported to Philadelphia for the centennial. "Its
aspect is ... faded and time-worn," the Ledger noted on May 8
of that year. "The text is fully legible, but the major part of the
signatures are so pale as to be only dimly discernible in the
strongest light ... and some are wholly invisible, the spaces which
contained them presenting only a blank."
Light is likely not the only reason the Declaration has become so
faded. In the early 1820s, an engraver made a facsimile possibly by
laying a piece of moist paper over the Declaration to soak up some
of the original ink. But light streaming through that Patent Office
window surely further reduced the legibility of our nation's most
precious handwritten document.
And fading of color or ink is only the most obvious injury that
light can cause. In the presence of oxygen or moisture or
temperature changes—or, worst of all, all three—light
can trigger chemical reactions that can degrade the very substance
of the parchment, paper, textile, or other material from which items
of artistic or historical interest are made. In fact, considering
the number of ways that light-induced chemical reactions can wreak
havoc, it's amazing that such items survive for any time at all.
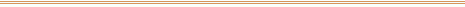 A bad reaction
A bad reaction
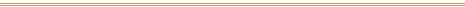
Light is paradoxically both our best friend and worst enemy when it
comes to enjoying our most beloved works of cultural heritage. For
it is light that makes visible the rich reds, oranges, and yellows
of a Turner sunset, say, or the deep blues of a Picasso blue-period
painting, or for that matter the dark brown ink gracing the
Declaration. We see those colors because they reflect light back to
our eye from very specific parts of the visible-light portion of the
electromagnetic spectrum.
Unfortunately, what's not reflected is absorbed, and that's where
the trouble starts. Light is energy, and when that absorbed energy
equals or exceeds the so-called activation energy of a molecule in a
dye, pigment, or ink—or in the paper or other material it
graces—the molecule becomes "excited," that is, rendered
available for chemical reactions.
"From a preservation standpoint, that's exactly what you don't
want," says Paul Messier, a Boston-based conservator of photographs
and works of art on paper. "If your object is chemically active, it
means it's interacting with the environment and becoming chemically
altered. Depending on the rate of those interactions, you've got a
recipe for poor preservation and a short life span."
Why? Because any number of things, many of them destructive, can
happen once a molecule gets excited. The extra energy may be
converted to heat (infrared energy) or emitted as light
(phosphorescence or fluorescence). It can break chemical bonds
within the molecule, creating smaller molecules and thereby
weakening the paper or parchment by shortening the long fibers that
make them strong.
Or it can jump to another molecule. In one of the most damaging of
such leaps, the energy transfers to an oxygen molecule, which can
then react with other molecules to jumpstart chemical reactions.
Such oxidation is the bane of museum curators, fine-art owners, and
all of us who want to make things last, whether they are national
treasures like the Declaration or a newspaper clipping of a personal
milestone.
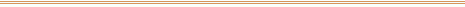 Adding insult to injury
Adding insult to injury
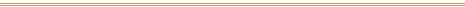
But that's not all. There can be synergistic effects: with higher
temperature and humidity, for example, reactions catalyzed by
electromagnetic radiation can occur more rapidly. And there can be
chain reactions: new substances formed as a result of photochemical
reactions will have enough energy to also react with the original
substance, launching a chain reaction of degradation.
Moreover, some kinds of light are more problematic than others.
Light toward the blue end of the visible spectrum, for example, is
higher frequency—and thus higher energy—than light at
the red end. Thus it packs a greater punch. And ultraviolet
radiation is more energetic still. In fact, it's the most damaging
type of electromagnetic energy in our everyday environment. Even
relatively low-energy infrared radiation can damage materials by
heating them and thereby helping to speed any chemical reactions
already under way.

A 50-watt incandescent light bulb spews out 100
billion billion photons a second, and 95 percent of those are
not helping us to see.

Conservators are concerned not only with frequency but with the
intensity of light hitting artworks or other
valuables—daylight, for instance, is typically brighter or
more intense than artificial light. And then there's exposure time.
As the Declaration shows so clearly, light damage is cumulative.
It's also irreversible (except, as one expert reminded me, in
PhotoShop). Finally, and arguably most unfair of all, chemical
reactions initiated by light can continue even after something is
placed in the dark.
 Substance abuse
Substance abuse
The range of our possessions at risk may be wider than you think. It
includes the media used to write, draw, and create photographs, such
as dyes, inks, pigments, varnishes, and oils, as well as the
materials they're used on, like paper, textiles, furniture,
feathers, fur, horn, and bone.
Some of these materials are at greater risk from light damage than
others. Generally speaking, organic materials—those derived
from plants or animals—are more susceptible than inorganic
materials. For instance, natural dyes, which are organic, generally
fade faster than pigments, which are usually comprised of inorganic
minerals. But even organic materials vary in their stability:
materials made of parchment, which is a specially prepared animal
skin, are less vulnerable than, say, silk and wool.
The Star-Spangled Banner is a case in point. Both the dyes and the
wool of our country's most famous flag have been seriously
light-degraded over time. And as expected, the red dye is more faded
than the blue. "The red dyes are more susceptible to fading because
they look red and thus absorb blue, and blue is the higher-energy
light," notes David Erhardt of the Smithsonian Center for Materials
Research and Education, who assisted the flag's conservation
project.
 Light housekeeping
Light housekeeping
What to do? Well, for starters, most art on display, whether in a
museum, gallery, or one's own home, is not being enjoyed at any
given time. "It's got all these photons smashing into it, and so for
every minute while it's being illuminated that nobody's looking at
it, that's all wasteful damage," says Steven Weintraub, a
conservator at Art Preservation Services in New York. "There are all
kinds of efficiencies [we can adopt] in terms of UV filters,
reducing the amount of light in general, and shutting off lights
when nobody is around to look at it."
One thing is to remove all those wavelengths we can't see anyway
from the light striking a valued object. A 50-watt incandescent
light bulb spews out 100
billion billion photons a second, and 95 percent of those are
either infrared or ultraviolet radiation, both of which are
invisible to us. All those unnecessary photons slamming into our
watercolor or framed photo at 186,000 miles per second are not
helping us to see; they're only helping to harm the item.
The National Archives filters light on the Declaration to exclude
higher-energy blue wavelengths, for instance. "You're looking
through multiple layers of glass at a very low light level, and that
light has had the harmful portion of the visible spectrum removed
from it as well as the ultraviolet," says Kitty Nicholson, a
conservator who worked on the Charters of Freedom project (see
A Conservative Approach). "So it's a very special light that both enhances the viewers'
visibility and also protects the document."
The National Archives also houses the Declaration in an oxygen-free
encasement filled with the inert gas argon, with carefully
controlled moisture and temperature. Some private institutions have
gone further still, storing vulnerable materials in such oxygen-free
enclosures in temperatures near or below freezing, in the dark. But
that would be pushing it for many cherished objects. As Messier
says, "What good is material that you can never see? It's all about
figuring out the balance."


|


|
John Hancock's famous signature dramatically reveals how
much the original Declaration (top) has faded versus the
Stone Engraving made in the 1820s.

|
|

|
In the electromagnetic spectrum, the shorter the
wavelength, the higher the energy level of the radiation
and thus the greater the damage inflicted on
light-sensitive materials.
|
|

|
This 19th-century photograph was affixed with a circular
price tag (to left of subject's mouth above) and sold at
an outdoor sale. When the new owner removed the tag, the
overall darkening of the image caused by that brief
exposure to sunlight was all too clear.

|
|

|
For over 3,000 years, these mural paintings in the tomb
of Nefertari in Egypt's Valley of the Queens were
protected from excessive light, moisture, and
temperature changes, hence their extraordinary
preservation.

|
|

|
Smithsonian conservators photograph the Star-Spangled
Banner during a recent conservation project.

|
|
|

